Table of Contents
Part 1
A recent Buzzfeed list has me questioning everything I thought I knew about how surgeons are trained on new medical devices, and how adequately the medical device reps themselves are trained on new products.
Part 2
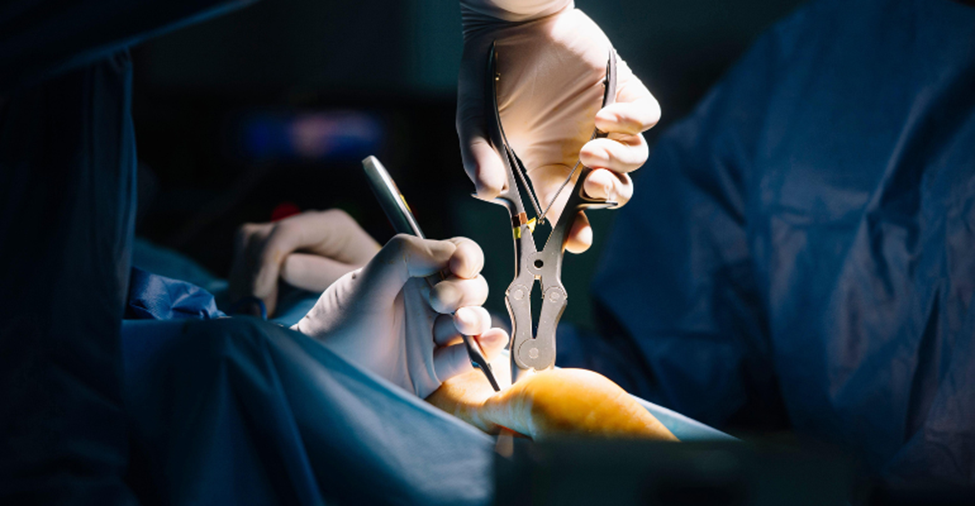
“Medical device rep here. You know that complicated spine surgery you just had? There’s a good chance that critical aspects of your care were performed by your surgeon using specialized tools for the very first time, and were directed from someone like me, in real time, with no special medical training, and with maybe a week of product training,” reads the first of 19 Caretaker Confessions.
It’s no secret that medical device reps often attend surgery and other medical procedures, and that they are allowed to provide information regarding the safe and effective use of their company’s products. After all, field reps are expected to be experts on the products they sell, and until now I’ve never really questioned whether or not medical device reps have been adequately trained on those products.
Part 3
“Often knowing more about their devices than the surgeons who use them, medical device sales representatives have therefore become commonplace in operating rooms across the United States,” Lisa Rice and Katie Stricklin, partners at Walsworth with expertise in life sciences litigation, writes in an article MD+DI published in 2017 that questions whether medical device sales reps in the operating room are an asset or a liability.
Part 4
According to Rice and Stricklin, manufacturers have seen an increase in products liability claims based upon their sales reps’ alleged representations or actions during medical procedures. “There is a fine line that, when crossed, turns a sales rep from an asset to both manufacturers and surgeons into a potential liability for third-party claims,” they write.
Such liability concerns are, in part, the reason most reputable medical device companies have corporate policies detailing expectations for field reps. Take Medtronic’s global business conduct standards policy for example. The policy states that Medtronic representatives “must not perform any clinical tasks or make any clinical decisions” and “must not touch patients or provide any patient care.” The policy also requires Medtronic representatives to “follow all applicable laws and regulations governing interactions with healthcare professionals.”
Part 5
But what if these policies are not enough to ensure patient safety and quality of care? What if some medical device reps are not properly trained on their products or on the ethical boundaries of their role? What if some surgeons rely too much on the guidance of medical device reps during surgery? What if some patients are unaware of the presence or influence of medical device reps in their procedures?
Audio
Follow Masco Medical
Part 6
These are some of the questions that I hope to explore in this blog post. I will share some insights from my own experience as a former medical device rep, as well as some research and opinions from experts in the field. I will also discuss some of the benefits and challenges of having medical device reps in the operating room, and some of the best practices for ensuring patient safety and quality of care.


Leave a Reply